The market for antibody drugs as innovative molecular-targeted drugs with fewer side effects has grown to be a 40 billion dollars industry worldwide.
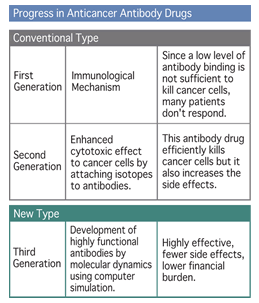
First generation anticancer antibody drugs mainly utilized immunological mechanisms to kill cancer cells. This method required many target molecules (diagram on the right ▼) on cancer cells and multiple bound of antibodies per cell. In spite of fewer side effects, many patients did not respond to this treatment. (Diagram 4)
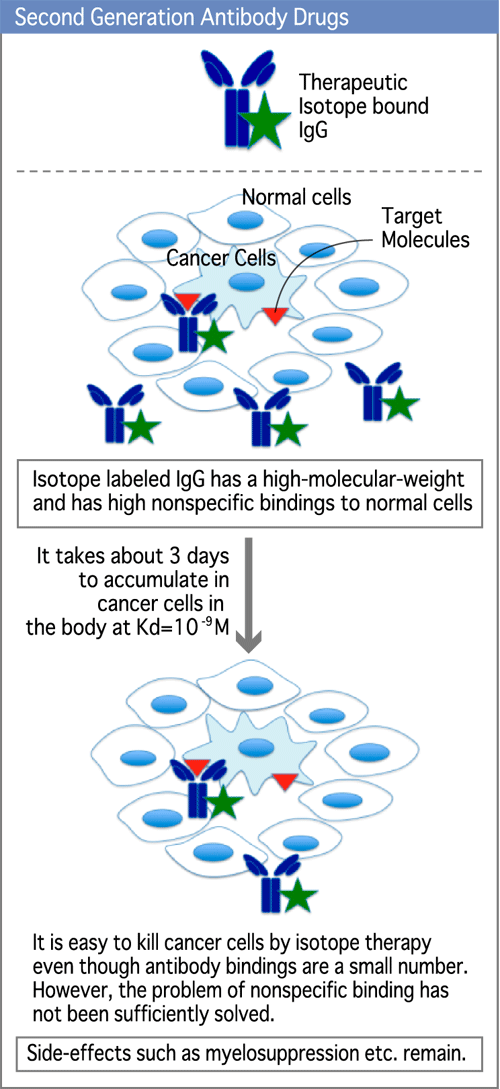
For second generation antibodies, isotopes were added to the antibodies to try to enhance the cytotoxic effect on cancer cells. However, it takes about 3 days for antibodies to bind to target antigens on cancer cells. In addition, nonspecific binding of the radioactive antibody produced more side effects caused by radiation (anemia, decrease in the white blood cell count, decrease in the platelet count, etc). (Diagram 5)
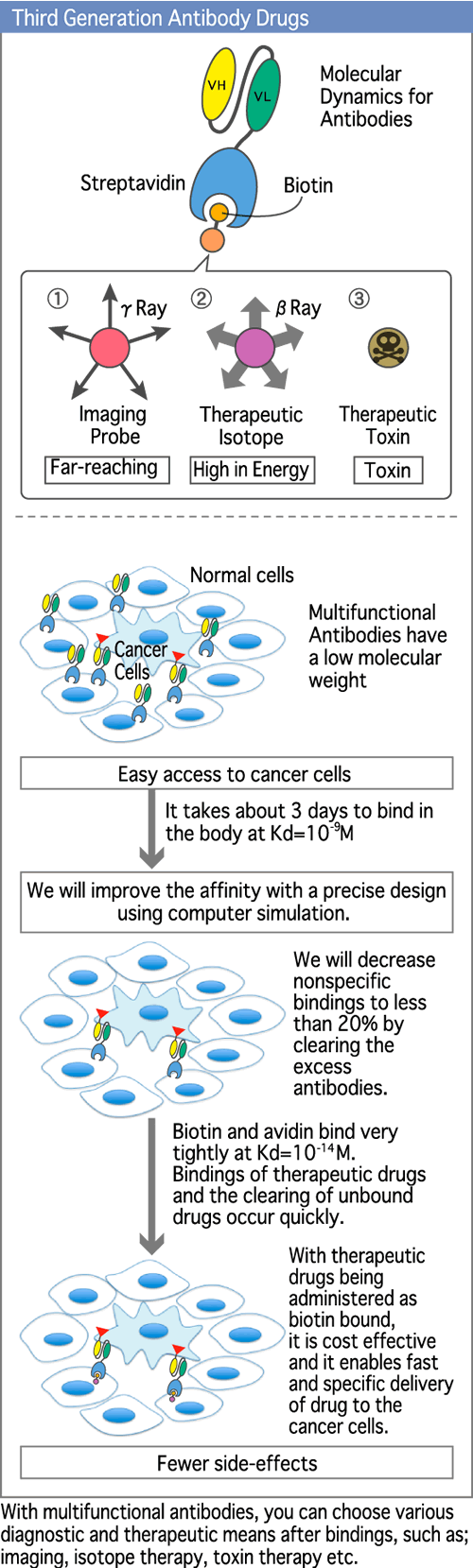
Now, we are developing third generation functional antibodies which are highly effective with fewer side effects. These third generation antibodies will also reduce the economic burden by taking advantage of computer simulations by molecular dynamics. (Diagram 3)
Specifically, we will isolate the domain of the monoclonal antibody which is involved in the recognition of the marker antigen on cancer cells from the domain involved in treatment (scFV). Streptavidin will be attached to scFv engineered antibodies and biotin will be attached to isotopes which kill cancer cells. The treatment of cancer cells is enhanced by injecting the engineered antibodies which will specifically bind onto cancer cells. Then, isotope labeled biotin will be injected. The radioactive biotin will selectively bind to the streptavidin-antibody-antigen complex on cancer cells to produce the cytotoxic effect. (Diagram 6)
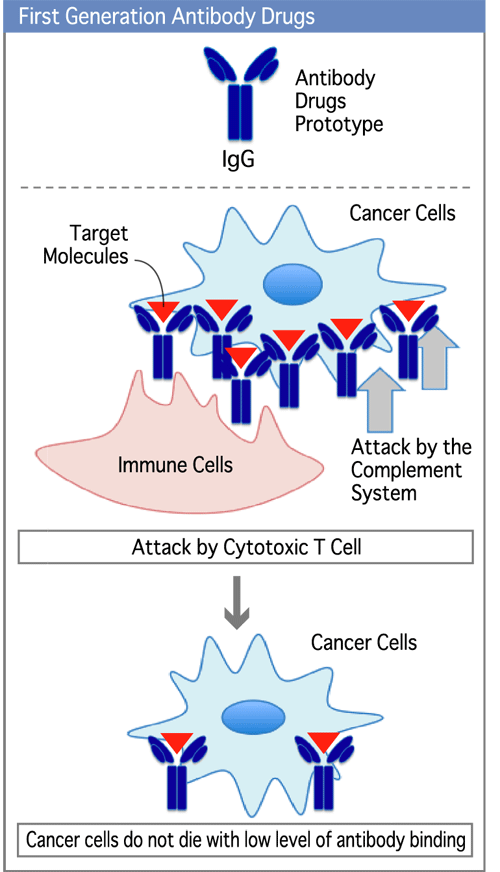
First generation anticancer antibody drugs mainly utilized immunological mechanisms to kill cancer cells. This method required many target molecules (diagram on the right ▼) on cancer cells and multiple bound of antibodies per cell. In spite of fewer side effects, many patients did not respond to this treatment. (Diagram 4)
For second generation antibodies, isotopes were added to the antibodies to try to enhance the cytotoxic effect on cancer cells. However, it takes about 3 days for antibodies to bind to target antigens on cancer cells. In addition, nonspecific binding of the radioactive antibody produced more side effects caused by radiation (anemia, decrease in the white blood cell count, decrease in the platelet count, etc). (Diagram 5)
Now, we are developing third generation functional antibodies which are highly effective with fewer side effects. These third generation antibodies will also reduce the economic burden by taking advantage of computer simulations by molecular dynamics. (Diagram 3)
Specifically, we will isolate the domain of the monoclonal antibody which is involved in the recognition of the marker antigen on cancer cells from the domain involved in treatment (scFV). Streptavidin will be attached to scFv engineered antibodies and biotin will be attached to isotopes which kill cancer cells. The treatment of cancer cells is enhanced by injecting the engineered antibodies which will specifically bind onto cancer cells. Then, isotope labeled biotin will be injected. The radioactive biotin will selectively bind to the streptavidin-antibody-antigen complex on cancer cells to produce the cytotoxic effect. (Diagram 6)



The third generation technology of the pre-targeting method was developed by Dr. Goldenberg and others. By attaching radioisotopes using the avidin-biotin reaction to pre-targeted antibodies on tumor cells, this method is useful for both imaging and treatment.
Although pre-targeting antibody drugs have produced promising results in the laboratory, none of these drugs have been approved for clinical use. Their major problems are low affinity of the antibody due to the scFV-SA fusion and the immunogenicity of streptavidin in patients. In addition, the technical difficulties in large scale production of modified monoclonal antibodies is also a major factor.
Among modified recombinant antibodies, the scFv is the most widely used and has the greatest potential for approval in clinical use. The scFv is the smallest recombinant antibody fragment constructed by connecting the variable domains of the heavy chain (VH) and the blight chain (VL), which are involved in antigen recognition, with an acidic linker. We produced a phage displaying the scFv, identified cancer cell-binding phages, and obtained a high number of anti-cancer antibodies from a phage display library.
Synthesized scFv antibodies show different distribution drug dynamics in our body from physiological antibodies. Furthermore, several problems still remain with scFV antibodies such as poor folding, a low level of expression, tendency to aggregate, and lack of stability. Dr. Tsumoto of the University of Tokyo developed a method to re-fold recombinant protein synthetized using the E. coli expression system to solve these problems
The rapid development of human scFv from mouse antibody genes that can be applied for diagnosis and treatment is essential.
Although pre-targeting antibody drugs have produced promising results in the laboratory, none of these drugs have been approved for clinical use. Their major problems are low affinity of the antibody due to the scFV-SA fusion and the immunogenicity of streptavidin in patients. In addition, the technical difficulties in large scale production of modified monoclonal antibodies is also a major factor.
Among modified recombinant antibodies, the scFv is the most widely used and has the greatest potential for approval in clinical use. The scFv is the smallest recombinant antibody fragment constructed by connecting the variable domains of the heavy chain (VH) and the blight chain (VL), which are involved in antigen recognition, with an acidic linker. We produced a phage displaying the scFv, identified cancer cell-binding phages, and obtained a high number of anti-cancer antibodies from a phage display library.
Synthesized scFv antibodies show different distribution drug dynamics in our body from physiological antibodies. Furthermore, several problems still remain with scFV antibodies such as poor folding, a low level of expression, tendency to aggregate, and lack of stability. Dr. Tsumoto of the University of Tokyo developed a method to re-fold recombinant protein synthetized using the E. coli expression system to solve these problems
The rapid development of human scFv from mouse antibody genes that can be applied for diagnosis and treatment is essential.